-
IP addresses are NOT logged in this forum so there's no point asking. Please note that this forum is full of homophobes, racists, lunatics, schizophrenics & absolute nut jobs with a smattering of geniuses, Chinese chauvinists, Moderate Muslims and last but not least a couple of "know-it-alls" constantly sprouting their dubious wisdom. If you believe that content generated by unsavory characters might cause you offense PLEASE LEAVE NOW! Sammyboy Admin and Staff are not responsible for your hurt feelings should you choose to read any of the content here. The OTHER forum is HERE so please stop asking.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
[COVID-19 Virus] The Sinkies are fucked Thread.
- Thread starter zhihau
- Start date
- Joined
- Jul 10, 2008
- Messages
- 35,666
- Points
- 113
I reckoned we are currently at the peak, these two days hiong hiong jit pai kwey.several weeks ago they said this omicorn wave would peak in several weeks. this is the peak or higher numbers expected ?
- Joined
- Jun 17, 2020
- Messages
- 13,772
- Points
- 113
They can say whatever they want.. The most important thing is, do pple believe them?several weeks ago they said this omicorn wave would peak in several weeks. this is the peak or higher numbers expected ?
Always the fault is Singaporeans, never the PAP. When infection rate came down, PAP always came to the forefront to take credit. It is a very standard protocol, no wonder Lee Hsien Yang said those white mice are very sanctimonious.
- Joined
- Jul 14, 2008
- Messages
- 90,068
- Points
- 113
The burning question on my mind is this- have we peaked?
Also another question is- can our missies tahan another 2~3 weeks before the cases drop to about a hundred or two?
The govt needs to reduce the number of people visiting clinics and hospitals for mild conditions immediately, whether it is to get MC or all-clear memo, to get "into the system", kiasu and kiasee or whatever reason. Most indications are that there are less serious cases of Omicron than Delta and the healthcare system should not be overloaded if not for those with mild symptoms.

- Joined
- Jul 10, 2008
- Messages
- 35,666
- Points
- 113
The folks go to loh kun choo because the clinics were fullThe govt needs to reduce the number of people visiting clinics and hospitals for mild conditions immediately, whether it is to get MC or all-clear memo, to get "into the system", kiasu and kiasee or whatever reason.



Protocol 1 is visit GP mah, MTF say one mah



- Joined
- Jul 14, 2008
- Messages
- 90,068
- Points
- 113
The folks go to loh kun choo because the clinics were full
Protocol 1 is visit GP mah, MTF say one mah
Another reason is that employers are giving some employees trouble over "unofficial MCs". This is something MOM and MOH need to sort out with the employers and reduce the confusion of simple sinkies.

- Joined
- Jul 10, 2008
- Messages
- 35,666
- Points
- 113
Absolutely spot on, some employees don’t have enough MC leaves to begin withAnother reason is that employers are giving some employees trouble over "unofficial MCs". This is something MOM and MOH need to sort out with the employers and reduce the confusion of simple sinkies.



- Joined
- Jul 14, 2008
- Messages
- 90,068
- Points
- 113
Absolutely spot on, some employees don’t have enough MC leaves to begin with
Btw I just saw this worrying report.

from msn.com:
10 children aged 5-11 had serious COVID vaccine side effects: HSA
INGAPORE — There were 10 children aged 5 to 11 years who have experienced serious side effects after being administered a COVID-19 vaccine, with symptoms including seizures (fits), appendicitis, allergic reactions and abnormal renal function reported.
However, this does not necessarily mean that the vaccine caused the serious adverse events (SAE) as they may be related to an underlying or undiagnosed disease, or it may be coincidental that they occurred around the same time that the vaccine was given, said the Health Sciences Authority (HSA).
No cases of myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the thin tissue sac around the heart) have been reported in this age group so far. "HSA is closely monitoring the AEs reported in children and is assessing them in the context of background incidence rates," added the agency.
In the latest vaccine safety update, published on Wednesday (23 February) and covering events up to end-January, HSA noted that some 280 AE reports for the 5 to 11 age group have been received so far. This represents 0.12 per cent out of 238,253 administered doses.
In comparison, the reporting rate of SAEs for that observed in adolescents and adults was 0.004 per cent of administered doses. The commonly reported AEs are also similar to that observed in adolescents (12-17 years old) and include angioedema (swelling of eyelids, face or lips), hives, dizziness, fever, rash, chest discomfort/pain, palpitations and shortness of breath.
Singapore began rolling out its vaccination programme to children aged 5-11 on 27 December 2021.
More than 15,000 AE reports

© Provided by Yahoo NewsINFOGRAPHIC: Health Sciences Authority
As of 31 January, HSA has received a total of 15,655 suspected AE reports, or 0.12 per cent of doses administered, associated with the use of Pfizer-BioNTech/Comirnaty and Moderna/Spikevax COVID-19 vaccines.
The most commonly reported AEs are consistent with those typically observed following vaccination such as with the flu vaccine. These include allergic reactions – such as rash, itch, hives and swelling of eyelids, face and lips – dizziness, shortness of breath, chest discomfort/pain, palpitations, fever, headache, muscle aches and injection site reactions such as pain and swelling.
These reported AEs generally resolved within a few days.
According to HSA, the reporting rate of serious AEs has remained stable between 0.004 per cent and 0.007 per cent. Of the 15,655 suspected AE reports received for the mRNA vaccines, 820 were assessed as serious.
Serious AEs comprised 0.006 per cent of doses administered. Of the reports received, many of the suspected AEs had resolved or were resolving at the time of reporting.
The most frequently reported serious AEs were anaphylaxis, or a severe and potentially life-threatening allergic reaction, at 88 reports and other severe allergic reactions (55 reports). Other serious AEs include:
- immunological – rheumatoid arthritis and other autoimmune conditions
- cardiovascular – chest pain, drop or increase in blood pressure, irregular heartbeat, tachycardia (fast heart rhythm), myocarditis and pericarditis
- neurological – migraine, nerve damage or dysfunction resulting in numbness or tingling/pricking sensation, muscle or limb weakness and pain in the affected area, syncope, seizures (fits), inflammation of the brain tissues, Bell’s Palsy (facial muscle weakness or paralysis) and cerebral venous thrombosis (CVT)
- haematological involving the blood cells - low platelets and blood clots
- musculoskeletal - joint inflammation or pain and muscle injury
- dermatological - severe skin reactions, eczema flare and skin blisters
- renal - reduced kidney function and inflammation of the kidney
- visual inflammation and visual disturbances
- tinnitus (ringing in the ears) and hearing loss
- respiratory - exacerbation of underlying asthma and breathing difficulties
- Other SAEs such as increase in liver enzymes, appendicitis, thyroid gland dysfunction, menstrual disorders, and infections.
- Joined
- Apr 14, 2011
- Messages
- 17,941
- Points
- 113
10 cases of serious adverse events reported in children aged 5 to 11 after COVID-19 vaccine jab: HSA
A girl holding on to her mother while getting a vaccine shot on Dec 27, 2021. (Photo: CNA/Hanidah Amin)
SINGAPORE: Ten cases of serious adverse events have been reported in children aged five to 11 who have taken their COVID-19 vaccine as of end-January, said the Health Sciences Authority (HSA) on Wednesday (Feb 23).
These reports included seizures, appendicitis, drop in blood pressure, allergic reaction, abnormal renal function and swelling of small blood vessels, said HSA. So far, no cases of myocarditis or pericarditis have been reported in this age group.
The COVID-19 vaccination programme was rolled out to children aged five to 11 on Dec 27 and the Pfizer-BioNTech/Comirnaty dose is the only approved vaccine for this age group.
As of end-January, 238,253 doses have been administered to children in this age group. The reporting rate of serious adverse events in this age group is at 0.004 per cent of the administered doses, said HSA.
The authority added, however, that the reports do not "necessarily mean that the vaccine has caused these serious (adverse events) as they may be related to an underlying or undiagnosed disease or it may be coincidental that they occurred around the same time that the vaccine was given".
"HSA is closely monitoring the (adverse events) reported in children and is assessing them in the context of background incidence rates," it added.
In all, 280 adverse events have been reported in this age group, representing 0.12 per cent of the doses that were administered. Common side effects include swelling of eyelids, face or lips, dizziness, fever and shortness of breath.
This is the 10th such COVID-19 vaccine safety update, covering the period since the roll-out of the national programme on Dec 30, 2020 to Jan 31, 2022.
Related:
MRNA VACCINES
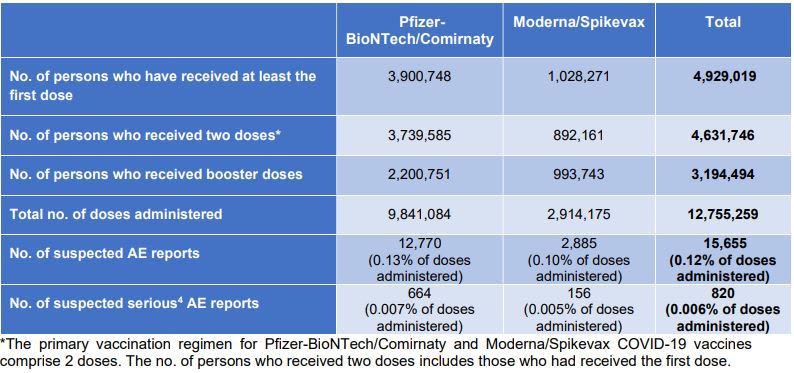
As of end-January, Singapore has administered 12,755,259 doses of the Pfizer-BioNTech/Comirnaty and Moderna/Spikevax mRNA vaccines, out of which 15,655 suspected adverse events reports were received.The most common side effects included allergic reactions such as rash or swelling of eyelids, face and lips, dizziness, shortness of breath and fever.
There were also 820 serious adverse events reported, including 88 cases of anaphylaxis and other reactions such as rheumatoid arthritis, severe skin reactions and low platelets and blood clots.
"HSA has assessed that the reporting rate of AEs and serious AEs of 0.12 per cent and 0.006 per cent of administered doses, respectively have remained stable since the roll-out of the COVID-19 vaccines and the overall benefits of the COVID-19 vaccines in preventing COVID-19 and serious complications associated with COVID-19 far outweigh any currently known AEs," said the authority.
NON-MRNA VACCINES
As of Jan 31, a total of 369,083 doses of the Sinovac-CoronaVac vaccine have been administered, with 299 suspected adverse events and 22 serious adverse events reported.For Sinopharm, 89,350 doses have been administered, with 41 suspected adverse events and six serious adverse events reported.
"The type and number of reports received for different COVID-19 vaccines are not directly comparable as the vaccines have been used in the vaccination programme for different durations of time," said HSA.

BOOSTER DOSES
Since the booster programme was rolled out on Sep 15, a total of 3,194,494 have received these jabs as of Jan 31, said HSA.In this category, there were 553 adverse events reports (0.03 per cent of doses administered) associated with the use of the PfizerBioNTech/Comirnaty vaccine and 289 such reports (0.03 per cent of doses administered) with the Moderna/Spikevax vaccine.
There were also 73 serious adverse event reports (0.002 per cent of administered doses), of which 15 cases were myocarditis and pericarditis - inflammation of the heart muscles and outer lining of the heart respectively.
ADVERSE EVENTS OF SPECIAL INTEREST
HSA said that it is closely monitoring several adverse events of special interest, including anaphylaxis, myocarditis, pericarditis and cerebral venous thrombosis.So far, the incidence rate of anaphylaxis reported locally with the vaccines has remained "low and stable" at about 0.89 per 100,000 doses administered, said HSA.
Of the 88 cases of anaphylaxis reported with the mRNA vaccines, all the patients recovered after medical treatment, said the authority.
It added that the number of cases of anaphylaxis associated with the second dose was lower than with the first dose of mRNA vaccines. To date, HSA has not received any cases of anaphylaxis associated with the booster dose.
For myocarditis and pericarditis, HSA said that it has as of end-January received 115 reports following the administration of mRNA vaccines. These cases happen more frequently in younger males below 30 years old, and more often with the second dose, said HSA.
One case of myocarditis with Sinovac-Coronavac vaccine has also been reported.
"Most cases are mild, with individuals reported to have recovered or are recovering," said the authority.
"It should be noted that COVID-19 infection is also known to be associated with myocarditis," HSA added.
There have been rare cases of cerebral venous thrombosis (CVT), or blood clots occurring in the veins of the brain, reported with the mRNA vaccines.
As of end-January, HSA has received 13 suspected reports of cerebral venous thrombosis with such vaccines.
"Vaccines are the best way to protect people from COVID-19 and have already saved many lives," said HSA.
"HSA’s current assessment is that the overall benefits of the Pfizer-BioNTech/Comirnaty, Moderna/Spikevax and Sinovac-CoronaVac COVID-19 vaccines in preventing COVID-19 and serious complications associated with COVID-19 far outweigh any currently known adverse events," it added.
Related:
- Joined
- Jul 14, 2008
- Messages
- 90,068
- Points
- 113
Latest report from HK via msn.com 
Daily COVID-19 cases and deaths continued to hit record levels in Hong Kong on Thursday as another infected child passed away.
According to the Hospital Authority, 50 people died on Wednesday. Their ages ranged from 52 to 97.
Only six received two doses of a COVID-19 vaccine. Two other were jabbed once.
Five of the 50 fatalities were 60 or younger. They all suffered from chronic illness.
Separately, the authority said a nine-year-old boy who had a chronic illness and was infected with COVID-19 died on Thursday.
He was found to be unconscious on Wednesday night and sent to the United Christian Hospital.
“His condition was very bad when he was admitted. He did not have a heart rate and could not breathe,” said Lau Ka-hin, the Hospital Authority’s chief manager (quality and standards).
Despite efforts to resuscitate him, the patient passed away at 7:07am on Thursday. He later tested positive for the coronavirus.
Lau said the boy was not vaccinated.
The chief manager added that the boy had a genetic muscular disease.
The case has been referred to the Coroner’s Court to determine the cause of death.
The authority also said there were 17 more deaths on Monday and Tuesday, which were not announced previously due to a delay in reporting. They were aged 68 to 93.
A total of 255 COVID-19 patients have died in public hospitals in the fifth wave. Most of them are elderly.
The Centre for Health Protection reported 8,798 new confirmed infections on Thursday, with all but three locally transmitted.
It was also notified by public and private facilities of 17,269 cases that are pending confirmation.
The Hospital Authority said 1,668 of its staff were infected with the virus.
To ease the pressures on the healthcare system, Lau said staff who tested positive or are close contacts of an infected person can inform their supervisor to arrange for them to return to work if they get a negative rapid antigen test result seven days later.
Lau also announced that around 300 beds in Tin Shui Wai Hospital will be converted for COVID-19 patients this Saturday.
He said this is to concentrate resources and manpower.
Lau said the hospital’s accident and emergency department will continue operating, but ambulances will only send those in emergency condition to the hospital.
Other patients will be sent to Tuen Mun Hospital.
Some patients currently in Tin Shui Wai Hospital will be transferred to other hospitals.
Patients visiting the hospital for outpatient services such as radiology and allied health services will be arranged to go to other hospitals or have their consultations via video calls.
Lau also appealed to COVID-19 patients with no or mild symptoms to patiently wait at home.
He said the Fire Services Department told the authority that for the past few days, more than 30 per cent of the people served by the ambulances were COVID-19 patients with no or mild symptoms.
This resulted in severe delays by ambulances for 358 cases.

Hong Kong sees record 50 daily COVID-19 deaths, 9-year-old boy who tested positive passes away
Daily COVID-19 cases and deaths continued to hit record levels in Hong Kong on Thursday as another infected child passed away.
According to the Hospital Authority, 50 people died on Wednesday. Their ages ranged from 52 to 97.
Only six received two doses of a COVID-19 vaccine. Two other were jabbed once.
Five of the 50 fatalities were 60 or younger. They all suffered from chronic illness.
Separately, the authority said a nine-year-old boy who had a chronic illness and was infected with COVID-19 died on Thursday.
He was found to be unconscious on Wednesday night and sent to the United Christian Hospital.
“His condition was very bad when he was admitted. He did not have a heart rate and could not breathe,” said Lau Ka-hin, the Hospital Authority’s chief manager (quality and standards).
Despite efforts to resuscitate him, the patient passed away at 7:07am on Thursday. He later tested positive for the coronavirus.
Lau said the boy was not vaccinated.
The chief manager added that the boy had a genetic muscular disease.
The case has been referred to the Coroner’s Court to determine the cause of death.
The authority also said there were 17 more deaths on Monday and Tuesday, which were not announced previously due to a delay in reporting. They were aged 68 to 93.
A total of 255 COVID-19 patients have died in public hospitals in the fifth wave. Most of them are elderly.
The Centre for Health Protection reported 8,798 new confirmed infections on Thursday, with all but three locally transmitted.
It was also notified by public and private facilities of 17,269 cases that are pending confirmation.
The Hospital Authority said 1,668 of its staff were infected with the virus.
To ease the pressures on the healthcare system, Lau said staff who tested positive or are close contacts of an infected person can inform their supervisor to arrange for them to return to work if they get a negative rapid antigen test result seven days later.
Lau also announced that around 300 beds in Tin Shui Wai Hospital will be converted for COVID-19 patients this Saturday.
He said this is to concentrate resources and manpower.
Lau said the hospital’s accident and emergency department will continue operating, but ambulances will only send those in emergency condition to the hospital.
Other patients will be sent to Tuen Mun Hospital.
Some patients currently in Tin Shui Wai Hospital will be transferred to other hospitals.
Patients visiting the hospital for outpatient services such as radiology and allied health services will be arranged to go to other hospitals or have their consultations via video calls.
Lau also appealed to COVID-19 patients with no or mild symptoms to patiently wait at home.
He said the Fire Services Department told the authority that for the past few days, more than 30 per cent of the people served by the ambulances were COVID-19 patients with no or mild symptoms.
This resulted in severe delays by ambulances for 358 cases.
- Joined
- Oct 12, 2021
- Messages
- 1,453
- Points
- 83
That is because you are the smartest person in the world.
u not paying attention to posts by leongSam the good burmese . i repeat here for u , maybe not in the exact same words.
covid is NOT deadly , mask , safe distancing , vaccine DO NOT work. u use mask only if u want to rob bank , u do safe distancing only when u are
surrounded by ah neh or if you win toto first prize. vaccine is absolutely a hoax.
covid is deadly to those with UNDERLYING medical problem. for those with multiple or server medical condition ,there is no cure.
people suffering from mild medical condition may be cure by ivermectin , vit D
taking Vit D and zinc definitely reduced the chance of getting infected to 0.01%
- Joined
- Jul 14, 2008
- Messages
- 90,068
- Points
- 113
u not paying attention to posts by leongSam the good burmese . i repeat here for u , maybe not in the exact same words.
covid is NOT deadly , mask , safe distancing , vaccine DO NOT work. u use mask only if u want to rob bank , u do safe distancing only when u are
surrounded by ah neh or if you win toto first prize. vaccine is absolutely a hoax.
covid is deadly to those with UNDERLYING medical problem. for those with multiple or server medical condition ,there is no cure.
people suffering from mild medical condition may be cure by ivermectin , vit D
taking Vit D and zinc definitely reduced the chance of getting infected to 0.01%
Safe distancing is the best invention since sliced bread.

- Joined
- Apr 14, 2011
- Messages
- 17,941
- Points
- 113
Some companies insist on Covid-19-infected staff getting MC despite authorities saying it's not necessary

Patients are seen outside a clinic in Tiong Bahru on Feb 25, 2022.
- Some firms still insist on their staff producing a medical certificate, if they self-test positive for Covid-19
- Such employers cite the need to "verify" their workers are truly sick
- Others say their company policy requires such documentation before staff are entitled to paid sick leave
- Some workers, while not explicitly mandated to take MC, still do so for various reasons
BY
NABILAH AWANG
BY
TAUFIQ ZALIZAN
Published February 27, 2022Updated February 27, 2022
WhatsAppTelegramFacebookTwitterEmailLinkedIn
SINGAPORE — Some companies are still insisting on their staff getting medical certificates (MC) from doctors if they are infected with Covid-19, despite the authorities stating that mild or asymptomatic cases do not require such documentation to be absent from work.
Among firms that do not make producing an MC a strict requirement, some workers still feel compelled to do so due to various conditions imposed by their bosses, such as making them work from home while they are down with the coronavirus.
ADVERTISEMENT
But most of the employers or employees that TODAY reached out to said that their workplaces do not require staff to produce an MC at all, despite being in manpower-reliant sectors such as food and beverage (F&B) or security.
All employees whom TODAY spoke to requested for their identities to be hidden, either because they were not authorised to speak to the media, or out of concerns of reprisal from their supervisors.
NO MC, NO PAID SICK LEAVE
READ ALSO
MOH urges public not to further strain healthcare system, as S'pore reports over 26,000 new Covid-19 cases
One hotel employee said that when she self-tested positive Covid-19 recently, her boss immediately told her to see a doctor to get an MC to be entitled to paid sick leave, citing company policy.
Even after she showed recent guidelines by the Ministry of Health, her supervisor did not budge.
ADVERTISEMENT
Mr Elson Lee, founder and chief executive of a student recruitment company, said he insists on seeing an MC to verify an employee’s claim that he is Covid-19 positive.
“How would we know if the employee is genuinely unwell? Like how do we verify, do we call MOH? Even that is a big hassle,” he said, adding that employees should be made to take a supervised antigen rapid test (ART) at a quick-test centre (QTC).
“I think the QTCs should send an alert to the HR department of the company, I think that’s more efficient,” he suggested.
Another employee at a technology firm who was recently infected with Covid-19 said that his employer had initially demanded to see an MC to approve his absence from work.
READ ALSO
'I want to be in the system': Patients with Covid-19 mild symptoms bog down GPs, polyclinics after self-test
His boss later begrudgingly allowed him to rest for “one to two days” and to work from home thereafter, despite him having a fever.
ADVERTISEMENT
“I went to take MC (eventually) so I have five days to rest. It’s a bit ridiculous,” said the employee.
On Feb 5, three government ministries and the Early Childhood Development Agency issued a joint statement, saying that many people with no or mild symptoms are visiting clinics just to obtain a letter or memo certifying that they have recovered from Covid-19 to support their return to work or to school.
“These visits are not necessary and risk compromising the standard of care for other patients who genuinely require medical attention,” they said.
The next day, the ministry reiterated on Facebook that people who tested positive for Covid-19 can safely recover at home if they are low risk and have mild or no symptoms.
It added that for these cases, there is "no need to see a doctor and get an MC", and employers and schools will accept a positive antigen rapid test result as proof of infection.
ADVERTISEMENT
READ ALSO
Healthcare system 'still holding up' despite Covid-19 count hitting 5 figures on some days, says Ong Ye Kung
RELIEF FOR DRIVERS WITHOUT MC
Meanwhile, some taxi and private-hire-car operators said that they minimally require drivers to take a supervised ART test at QTCs in order to qualify for rental waivers.
Ms Tammy Tan, group chief branding and communications officer at taxi operator ComfortDelGro, said waivers will be credited for unwell drivers who took MC or “are asymptomatic and had gone to a Quick Test Centre and received an SMS of their ART results”.
Mr Yeow How Peng, head of Strides Mobility Services, said the taxi operator accepts supporting documents of drivers' Covid-19 status, such as "MCs, MOH messages or ART results", in granting rental waivers.
Meanwhile, Grab and Gojek offer various additional support besides rental waivers for drivers who sign up for optional programmes or meet certain criteria.
“Active driver- and delivery-partners who have tested positive on self-administered ART or at Quick Test Centres are entitled to a one-time income support of up to S$500,” said a Grab spokesperson.
READ ALSO
No doctor memo on Covid-19 recovery needed for people to return to work or school: Govt
A Gojek spokesperson said that driver-partners who sign up for its Freelancer Earning Protection programme are entitled to daily payouts if they produce a positive result via a supervised ART test.
'HOW DO YOU EVEN CHAO KENG ART RESULT?'
SSA Culinary Institute, a culinary school, said that it stopped requiring staff to present an MC if they are infected with Covid-19.
Over the past month, five employees became Covid-19 positive. All of them merely had to snap photos of the positive result on the ART kits that the company provided for them.
“I find it irresponsible to expect employees to go to the doctor because they will risk infecting others when they are out of isolation,” said Ms Siti Humairah Suhaimi, the firm's assistant vice president of operations.
A restaurant at One Raffles Place similarly does not require further proof from staff besides a self-administered ART result.
This is despite the outlet running operations with only four full-time employees and two regular part-timers.
“We trust all our employees as we strongly believe that trust builds productivity,” said the restaurant's spokesperson.
Some companies that do not require MC proof are sticking to this policy despite the fact that their manpower has been affected by a recent rise in Covid-19 cases.
Aardvark Security Services' managing director Khairul Annuar Rudy Shahril said that just last weekend alone, the company had a dozen officers either put on Health Risk Notice or were themselves Covid-19 cases.
He said he did not see the need to make his officers visit a clinic solely for an MC to mitigate the risk of “chao keng” — local slang for malingering.
“If the guy doesn’t want to come to work, he will find other means not to come to work,” said Mr Khairul.
“Besides, how do you even chao keng your ART result?”
- Joined
- Oct 30, 2014
- Messages
- 36,768
- Points
- 113
SINGAPORE: Singapore reported 14,228 new COVID-19 cases as of noon on Sunday (Feb 27), comprising 14,064 local and 164 imported infections.
There were eight fatalities, taking the death toll from coronavirus complications to 1,007.
There are 1,553 patients in hospital, according to the latest infection statistics on the Ministry of Health (MOH) website. A total of 214 patients required oxygen supplementation.
Forty-six patients are in the intensive care unit, compared to 50 on Saturday.
https://www.channelnewsasia.com/singapore/covid-19-cases-feb-27-deaths-hospital-icu-2522856
- Joined
- Jul 10, 2008
- Messages
- 35,666
- Points
- 113
Hip hip hooray! Can stand down those restrictions already?SINGAPORE: Singapore reported 14,228 new COVID-19 cases as of noon on Sunday (Feb 27), comprising 14,064 local and 164 imported infections.
- Joined
- Apr 14, 2011
- Messages
- 17,941
- Points
- 113
Love it! Only 8 Jiak Liao Bee dead!
Similar threads
- Replies
- 3
- Views
- 175
- Replies
- 2
- Views
- 234
- Replies
- 5
- Views
- 563
- Replies
- 0
- Views
- 231
