- Joined
- Aug 8, 2008
- Messages
- 6,070
- Points
- 83
http://www.sohu.com/a/294759690_260616?spm=smpc.home.top-news4.1.1550171686156j7Yq715

澎湃新闻
16万文章 0总阅读
查看TA的文章>
31
疟原虫治晚期癌症志愿者招募已满:疗法处早期阶段,理性就医
2019-02-14 22:46
春节刷屏至今的“疟原虫治疗晚期癌症”项目有了新的动态,该项目由中科院广州生物医药与健康研究院研究员陈小平主导。
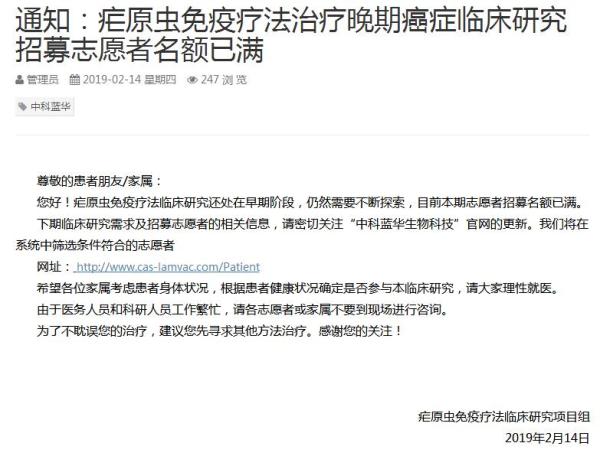
2月14日,澎湃新闻记者(www.thepaper.cn)在广州中科蓝华生物科技有限公司(下称“中科蓝华”)官网看到,网站发布一则通知:疟原虫免疫疗法治疗晚期癌症临床研究招募志愿者名额已满。
中国临床试验注册中心官网显示,中科蓝华为三项疟原虫治疗癌症相关项目的申请人所在单位、研究实施负责单位。该公司成立于2013年1月,注册资本1900万元,最大股东及实际控制人为柯宗贵,柯宗贵也是上市公司蓝盾信息安全技术股份有限公司(300297)的最大股东。陈小平为中科蓝华创始人,现任首席执行官(CEO)。
疟原虫免疫疗法临床研究项目组在通知中表示,疟原虫免疫疗法临床研究还处在早期阶段,仍然需要不断探索,目前本期志愿者招募名额已满。下期临床研究需求及招募志愿者的相关信息,请密切关注“中科蓝华生物科技”官网的更新。项目组将在系统中筛选条件符合的志愿者。
通知还写道,希望各位家属考虑患者身体状况,根据患者健康状况确定是否参与本临床研究,请大家理性就医。
另外,项目组称,由于医务人员和科研人员工作繁忙,请各志愿者或家属不要到现场进行咨询。并且,为了不耽误患者治疗,建议其先寻求其他方法治疗。
澎湃新闻还获悉,原定于2月14日、2月15日在广州复大肿瘤医院的两场疟原虫科研项目咨询也全部取消。
目前名额已满的这轮招募信息最早于1月31日在中科蓝华官网发布。患者招募信息称,疟原虫免疫疗法治疗晚期肺癌的临床研究目前已经在广州医科大学附属第一医院与钟南山院士的团队合作开展,取得了初期研究进展,现扩展到其它癌症中。
新招募患者的临床试验地点广州医科大学附属第一医院、云南昆钢医院(云南省昆明市第四人民院)、广州复大肿瘤医院。
澎湃新闻记者2月13日从患者家属中曾获悉,或将有第四家医院实施疟原虫治疗。陈小平团队称即将在广州医科大学附属第五医院启动疟原虫免疫疗法治疗中晚期实体瘤的临床研究。
中科蓝华方面的工作人员向家属介绍,计划是广州医科大学附属第一医院招收30例受试者,广州复大肿瘤医院招收20例受试者,云南昆钢医院招收90例受试者,广州医科大学附属第五医院招收30例受试者。
在中科蓝华患者招募网站上看到,志愿者年龄需在18-70岁,生活能够自理,饮食良好,癌症类别为非小细胞肺癌、肝/胆癌、胃癌、乳腺癌、结直肠癌这五种。 接受治疗的志愿者需要住院治疗2个月左右。
陈小平团队所谓的“疟原虫治疗晚期癌症”,即向符合入组条件的晚期癌症患者注射含有疟原虫(间日疟原虫)的红细胞。团队此前在小鼠动物实验看到,疟原虫感染能显著抑制小鼠肺癌(Lewis肺癌)的生长和转移,显著延长荷瘤小鼠的生存时间。团队随后还发文解释背后机制:癌症小鼠感染疟原虫之后,其免疫细胞会被激活,这些免疫细胞激活之后会杀死肿瘤细胞。与此同时,肿瘤组织中起到抑制抗肿瘤免疫反应的细胞也会被疟原虫感染所抑制,因而解放了肿瘤组织中的免疫抑制微环境,并促进T细胞进入到肿瘤中去,从而有效杀死肿瘤细胞。
陈小平团队自2016年起在医院正式开展疟原虫免疫疗法治疗晚期实体肿瘤的临床试验。直到1月28日,中科院官方微博发布消息,在中科院SELF论坛的一场公开演讲里,陈小平介绍了上述疗法,并称,团队研究发现肿瘤死亡率与疟疾发病率呈现负相关关系,疟原虫对治疗癌症有帮助,目前临床试验发现,10名病人中,有5人治疗效果明显,其中2人可能被治愈。
随后,疟原虫治疗晚期癌症的消息在网络上刷屏至今。不过,这种“以毒攻毒”、直接向患者注射病原体的治疗方式,也陆续引发一些业内人士的质疑。返回搜狐,查看更多
声明:该文观点仅代表作者本人,搜狐号系信息发布平台,搜狐仅提供信息存储空间服务。
澎湃News
160,000 articles
0 total reading
View TA's article >
31
share to
Plasmodium treatment of advanced cancer volunteers has been recruited: early stage of therapy, rational medical treatment
2019-02-14 22:46
The “Plasmodium Treatment of Advanced Cancer” project has been updated in the Spring Festival. The project is led by Chen Xiaoping, a researcher at the Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences.
On February 14th, the 澎湃 journalist (www.thepaper.cn) saw in the official website of Guangzhou Zhongke Lanhua Biotechnology Co., Ltd. (hereinafter referred to as “Zhongke Lanhua”) that the website issued a notice: Plasmodium immunotherapy treatment The number of volunteers recruited for advanced cancer clinical research is full.
According to the official website of the China Clinical Trial Registration Center, Zhongke Lanhua is the unit responsible for the three malaria parasites for cancer-related treatment projects and the responsible unit for research implementation. The company was established in January 2013 with a registered capital of 19 million yuan. The largest shareholder and actual controller is Ke Zonggui. Ke Zonggui is also the largest shareholder of the listed company Blue Shield Information Security Technology Co., Ltd. (300297). Chen Xiaoping is the founder of Zhongke Lanhua and the current CEO (CEO).
The Plasmodium Immunotherapy Clinical Research Project Team stated in the notice that the clinical study of Plasmodium immunotherapy is still in its early stages and still needs to be explored continuously. At present, the number of volunteer recruitment is full. For the next phase of clinical research needs and information on recruiting volunteers, please pay close attention to the update of the official website of Zhongke Lanhua Biotechnology. The project team will screen the volunteers who meet the criteria in the system.
The notice also wrote that I hope that all family members will consider the patient's physical condition and determine whether to participate in this clinical study according to the patient's health status. Please seek medical advice rationally.
In addition, the project team said that due to the busy work of medical staff and scientific researchers, volunteers or their families should not go to the site for consultation. Moreover, in order not to delay the treatment of patients, it is recommended to seek other methods of treatment.
The news also learned that the consultations on two malaria parasite research projects scheduled to be held at Guangzhou Fuda Cancer Hospital on February 14 and February 15 were all cancelled.
The current round of recruitment information has been published on the official website of Zhongke Lanhua on January 31. According to the patient recruitment information, the clinical study of Plasmodium immunotherapy for advanced lung cancer has been carried out in cooperation with the team of the First Affiliated Hospital of Guangzhou Medical University and Academician Zhong Nanshan, and has achieved initial research progress and is now expanding into other cancers.
The clinical trial site for newly recruited patients is the First Affiliated Hospital of Guangzhou Medical University, Yunnan Kunshan Iron and Steel Hospital (the Fourth People's Hospital of Kunming, Yunnan Province), and Guangzhou Fuda Cancer Hospital.
澎湃The journalist was informed on February 13 from the family of the patient that there would be a fourth hospital to implement the treatment of malaria parasites. Chen Xiaoping said that he will start the clinical study of Plasmodium immunotherapy in the treatment of advanced solid tumors in the Fifth Affiliated Hospital of Guangzhou Medical University.
The staff of Zhongke Lanhua introduced to the family that the plan is to enroll 30 subjects in the First Affiliated Hospital of Guangzhou Medical University, to recruit 20 subjects from Guangzhou Fuda Cancer Hospital, and to enroll 90 subjects in Yunnan Kunshan Iron and Steel Hospital. The fifth hospital affiliated to Guangzhou Medical University enrolled 30 subjects.
On the recruitment website of Zhongke Lanhua patient, the volunteers need to be 18-70 years old, and they can take care of themselves and have a good diet. The cancer categories are non-small cell lung cancer, liver/chosal cancer, stomach cancer, breast cancer and colorectal cancer. These five kinds. Volunteers receiving treatment need to be hospitalized for about 2 months.
Chen Xiaoping’s team called “Plasmodium to treat advanced cancer”, which is to inject red blood cells containing Plasmodium (P. vivax) into patients with advanced cancer who meet the enrollment criteria. The team previously observed in mouse animal experiments that Plasmodium infection significantly inhibited the growth and metastasis of mouse lung cancer (Lewis lung cancer), significantly prolonging the survival time of tumor-bearing mice. The team then issued a document explaining the underlying mechanism: after cancer mice are infected with Plasmodium, their immune cells are activated, and these immune cells activate tumor cells after activation. At the same time, cells that inhibit the anti-tumor immune response in tumor tissues are also inhibited by Plasmodium infection, thus liberating the immunosuppressive microenvironment in tumor tissues and promoting T cells to enter the tumor, thereby effectively killing Dead tumor cells.
Chen Xiaoping’s team has officially started clinical trials of Plasmodium immunotherapy for advanced solid tumors in hospital since 2016. Until January 28th, the official microblog of the Chinese Academy of Sciences released a message. In a public speech at the SELF Forum of the Chinese Academy of Sciences, Chen Xiaoping introduced the above therapy and said that the team study found a negative correlation between tumor mortality and malaria incidence, malaria parasites. It is helpful for the treatment of cancer. At present, clinical trials have found that 5 out of 10 patients have obvious treatment effects, and 2 of them may be cured.
Subsequently, the news that Plasmodium treats advanced cancer has been screened on the Internet to this day. However, this "drug-to-drug" treatment of direct injection of pathogens into patients has also raised questions from some insiders. Go back to Sohu and see more
Disclaimer: This article only represents the author himself, Sohu is the information publishing platform, and Sohu only provides information storage space services.
澎湃新闻
16万文章 0总阅读
查看TA的文章>
31
- 分享到
疟原虫治晚期癌症志愿者招募已满:疗法处早期阶段,理性就医
2019-02-14 22:46
春节刷屏至今的“疟原虫治疗晚期癌症”项目有了新的动态,该项目由中科院广州生物医药与健康研究院研究员陈小平主导。
2月14日,澎湃新闻记者(www.thepaper.cn)在广州中科蓝华生物科技有限公司(下称“中科蓝华”)官网看到,网站发布一则通知:疟原虫免疫疗法治疗晚期癌症临床研究招募志愿者名额已满。

中国临床试验注册中心官网显示,中科蓝华为三项疟原虫治疗癌症相关项目的申请人所在单位、研究实施负责单位。该公司成立于2013年1月,注册资本1900万元,最大股东及实际控制人为柯宗贵,柯宗贵也是上市公司蓝盾信息安全技术股份有限公司(300297)的最大股东。陈小平为中科蓝华创始人,现任首席执行官(CEO)。
疟原虫免疫疗法临床研究项目组在通知中表示,疟原虫免疫疗法临床研究还处在早期阶段,仍然需要不断探索,目前本期志愿者招募名额已满。下期临床研究需求及招募志愿者的相关信息,请密切关注“中科蓝华生物科技”官网的更新。项目组将在系统中筛选条件符合的志愿者。
通知还写道,希望各位家属考虑患者身体状况,根据患者健康状况确定是否参与本临床研究,请大家理性就医。
另外,项目组称,由于医务人员和科研人员工作繁忙,请各志愿者或家属不要到现场进行咨询。并且,为了不耽误患者治疗,建议其先寻求其他方法治疗。
澎湃新闻还获悉,原定于2月14日、2月15日在广州复大肿瘤医院的两场疟原虫科研项目咨询也全部取消。
目前名额已满的这轮招募信息最早于1月31日在中科蓝华官网发布。患者招募信息称,疟原虫免疫疗法治疗晚期肺癌的临床研究目前已经在广州医科大学附属第一医院与钟南山院士的团队合作开展,取得了初期研究进展,现扩展到其它癌症中。
新招募患者的临床试验地点广州医科大学附属第一医院、云南昆钢医院(云南省昆明市第四人民院)、广州复大肿瘤医院。
澎湃新闻记者2月13日从患者家属中曾获悉,或将有第四家医院实施疟原虫治疗。陈小平团队称即将在广州医科大学附属第五医院启动疟原虫免疫疗法治疗中晚期实体瘤的临床研究。
中科蓝华方面的工作人员向家属介绍,计划是广州医科大学附属第一医院招收30例受试者,广州复大肿瘤医院招收20例受试者,云南昆钢医院招收90例受试者,广州医科大学附属第五医院招收30例受试者。
在中科蓝华患者招募网站上看到,志愿者年龄需在18-70岁,生活能够自理,饮食良好,癌症类别为非小细胞肺癌、肝/胆癌、胃癌、乳腺癌、结直肠癌这五种。 接受治疗的志愿者需要住院治疗2个月左右。
陈小平团队所谓的“疟原虫治疗晚期癌症”,即向符合入组条件的晚期癌症患者注射含有疟原虫(间日疟原虫)的红细胞。团队此前在小鼠动物实验看到,疟原虫感染能显著抑制小鼠肺癌(Lewis肺癌)的生长和转移,显著延长荷瘤小鼠的生存时间。团队随后还发文解释背后机制:癌症小鼠感染疟原虫之后,其免疫细胞会被激活,这些免疫细胞激活之后会杀死肿瘤细胞。与此同时,肿瘤组织中起到抑制抗肿瘤免疫反应的细胞也会被疟原虫感染所抑制,因而解放了肿瘤组织中的免疫抑制微环境,并促进T细胞进入到肿瘤中去,从而有效杀死肿瘤细胞。
陈小平团队自2016年起在医院正式开展疟原虫免疫疗法治疗晚期实体肿瘤的临床试验。直到1月28日,中科院官方微博发布消息,在中科院SELF论坛的一场公开演讲里,陈小平介绍了上述疗法,并称,团队研究发现肿瘤死亡率与疟疾发病率呈现负相关关系,疟原虫对治疗癌症有帮助,目前临床试验发现,10名病人中,有5人治疗效果明显,其中2人可能被治愈。
随后,疟原虫治疗晚期癌症的消息在网络上刷屏至今。不过,这种“以毒攻毒”、直接向患者注射病原体的治疗方式,也陆续引发一些业内人士的质疑。返回搜狐,查看更多
声明:该文观点仅代表作者本人,搜狐号系信息发布平台,搜狐仅提供信息存储空间服务。
澎湃News
160,000 articles
0 total reading
View TA's article >
31
share to
Plasmodium treatment of advanced cancer volunteers has been recruited: early stage of therapy, rational medical treatment
2019-02-14 22:46
The “Plasmodium Treatment of Advanced Cancer” project has been updated in the Spring Festival. The project is led by Chen Xiaoping, a researcher at the Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences.
On February 14th, the 澎湃 journalist (www.thepaper.cn) saw in the official website of Guangzhou Zhongke Lanhua Biotechnology Co., Ltd. (hereinafter referred to as “Zhongke Lanhua”) that the website issued a notice: Plasmodium immunotherapy treatment The number of volunteers recruited for advanced cancer clinical research is full.
According to the official website of the China Clinical Trial Registration Center, Zhongke Lanhua is the unit responsible for the three malaria parasites for cancer-related treatment projects and the responsible unit for research implementation. The company was established in January 2013 with a registered capital of 19 million yuan. The largest shareholder and actual controller is Ke Zonggui. Ke Zonggui is also the largest shareholder of the listed company Blue Shield Information Security Technology Co., Ltd. (300297). Chen Xiaoping is the founder of Zhongke Lanhua and the current CEO (CEO).
The Plasmodium Immunotherapy Clinical Research Project Team stated in the notice that the clinical study of Plasmodium immunotherapy is still in its early stages and still needs to be explored continuously. At present, the number of volunteer recruitment is full. For the next phase of clinical research needs and information on recruiting volunteers, please pay close attention to the update of the official website of Zhongke Lanhua Biotechnology. The project team will screen the volunteers who meet the criteria in the system.
The notice also wrote that I hope that all family members will consider the patient's physical condition and determine whether to participate in this clinical study according to the patient's health status. Please seek medical advice rationally.
In addition, the project team said that due to the busy work of medical staff and scientific researchers, volunteers or their families should not go to the site for consultation. Moreover, in order not to delay the treatment of patients, it is recommended to seek other methods of treatment.
The news also learned that the consultations on two malaria parasite research projects scheduled to be held at Guangzhou Fuda Cancer Hospital on February 14 and February 15 were all cancelled.
The current round of recruitment information has been published on the official website of Zhongke Lanhua on January 31. According to the patient recruitment information, the clinical study of Plasmodium immunotherapy for advanced lung cancer has been carried out in cooperation with the team of the First Affiliated Hospital of Guangzhou Medical University and Academician Zhong Nanshan, and has achieved initial research progress and is now expanding into other cancers.
The clinical trial site for newly recruited patients is the First Affiliated Hospital of Guangzhou Medical University, Yunnan Kunshan Iron and Steel Hospital (the Fourth People's Hospital of Kunming, Yunnan Province), and Guangzhou Fuda Cancer Hospital.
澎湃The journalist was informed on February 13 from the family of the patient that there would be a fourth hospital to implement the treatment of malaria parasites. Chen Xiaoping said that he will start the clinical study of Plasmodium immunotherapy in the treatment of advanced solid tumors in the Fifth Affiliated Hospital of Guangzhou Medical University.
The staff of Zhongke Lanhua introduced to the family that the plan is to enroll 30 subjects in the First Affiliated Hospital of Guangzhou Medical University, to recruit 20 subjects from Guangzhou Fuda Cancer Hospital, and to enroll 90 subjects in Yunnan Kunshan Iron and Steel Hospital. The fifth hospital affiliated to Guangzhou Medical University enrolled 30 subjects.
On the recruitment website of Zhongke Lanhua patient, the volunteers need to be 18-70 years old, and they can take care of themselves and have a good diet. The cancer categories are non-small cell lung cancer, liver/chosal cancer, stomach cancer, breast cancer and colorectal cancer. These five kinds. Volunteers receiving treatment need to be hospitalized for about 2 months.
Chen Xiaoping’s team called “Plasmodium to treat advanced cancer”, which is to inject red blood cells containing Plasmodium (P. vivax) into patients with advanced cancer who meet the enrollment criteria. The team previously observed in mouse animal experiments that Plasmodium infection significantly inhibited the growth and metastasis of mouse lung cancer (Lewis lung cancer), significantly prolonging the survival time of tumor-bearing mice. The team then issued a document explaining the underlying mechanism: after cancer mice are infected with Plasmodium, their immune cells are activated, and these immune cells activate tumor cells after activation. At the same time, cells that inhibit the anti-tumor immune response in tumor tissues are also inhibited by Plasmodium infection, thus liberating the immunosuppressive microenvironment in tumor tissues and promoting T cells to enter the tumor, thereby effectively killing Dead tumor cells.
Chen Xiaoping’s team has officially started clinical trials of Plasmodium immunotherapy for advanced solid tumors in hospital since 2016. Until January 28th, the official microblog of the Chinese Academy of Sciences released a message. In a public speech at the SELF Forum of the Chinese Academy of Sciences, Chen Xiaoping introduced the above therapy and said that the team study found a negative correlation between tumor mortality and malaria incidence, malaria parasites. It is helpful for the treatment of cancer. At present, clinical trials have found that 5 out of 10 patients have obvious treatment effects, and 2 of them may be cured.
Subsequently, the news that Plasmodium treats advanced cancer has been screened on the Internet to this day. However, this "drug-to-drug" treatment of direct injection of pathogens into patients has also raised questions from some insiders. Go back to Sohu and see more
Disclaimer: This article only represents the author himself, Sohu is the information publishing platform, and Sohu only provides information storage space services.












