mRNA vaccines are better..
'No evidence' inactivated virus vaccines more efficacious against COVID-19 variants than mRNA ones: Singapore expert committee
COVID-19 vaccination at Tanjong Pagar, Singapore (4)
A person receiving a dose of the COVID-19 vaccine at Tanjong Pagar Community Centre on Jan 27, 2021. (Photo: Jeremy Long)
Bookmark
SINGAPORE: There is "no evidence" that inactivated virus vaccines demonstrate higher efficacy against COVID-19 variants than messenger RNA (mRNA)-based vaccines, said the government-appointed expert committee on COVID-19 vaccination in Singapore.
"We have noted social media messages asserting that the mRNA COVID-19 vaccines are ineffective against (variants of concern) and that inactivated virus COVID-19 vaccines would provide superior protection," said the committee in a media release on Monday (Jun 7).
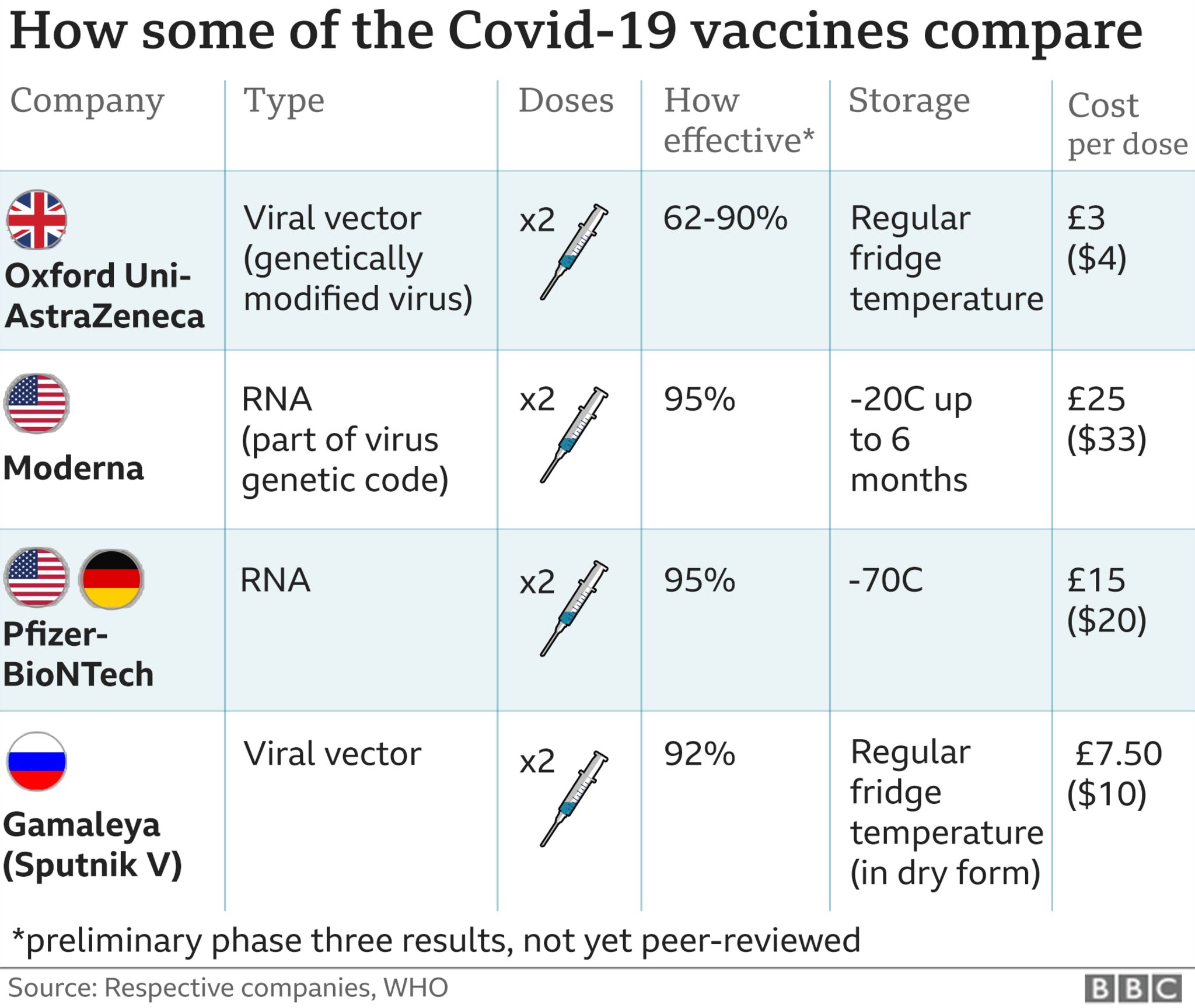
The two vaccines currently used in Singapore's national vaccination programme - Pfizer-BioNTech and Moderna - are based on mRNA technology. Both were recommended by the expert committee and authorised by the Health Science Authority (HSA) under the Pandemic Special Access Route (PSAR).
The committee said its assessment, based on a continual review of data and evidence, remains that the authorised mRNA vaccines are "safe and highly effective" and continue to show protection against the virus variants.
"The inactivated virus COVID-19 vaccines have variable protection and there is currently no evidence to suggest that inactivated virus vaccines demonstrate higher vaccine efficacy against (variants of concerns) than the mRNA vaccines," said the committee.
The Sinovac vaccine, of which Singapore has 200,000 doses, uses inactivated viruses.
Monday's clarification by the expert committee follows a similar statement less than three weeks ago, in response to an open letter by 12 doctors that had called for children to be given COVID-19 vaccines other than mRNA ones over fears of "unknown and unstudied" long-term side effects.
Eleven of the 12 doctors later retracted their statements.
AUTHORISED VACCINES "SAFE AND EFFECTIVE" AGAINST COVID-19
Both the Pfizer-BioNTech and Moderna vaccines have shown to be highly efficacious, especially in protecting against severe COVID-19 disease and hospitalisation, said the committee.
It said the high efficacy of the vaccines was demonstrated in Phase 3 clinical trials, and further supported with data from real-world rollouts of the vaccines, including in the United States, the UK and Israel.
This also includes protection against key COVID-19 variants such as the Alpha and Beta variants, which were the predominant strains circulating in these countries. The Alpha variant, B117, was first detected in the UK while the Beta variant, B1351, was first detected in South Africa.
Infographic Greek names for COVID-19 variants B1617
The committee added that despite concerns over the Delta variant, which was first detected in India, data showed that the mRNA vaccines continue to be effective.
It cited a study in the UK, which showed that two doses of the Pfizer-BioNTech COVID-19 vaccine gave about 88 per cent protection against symptomatic COVID-19, even with the Delta variant.
"While further studies are required before a definitive conclusion can be made, the available data globally indicates that substantial protection is preserved," said the committee.
Commentary: Misinformation threatens Singapore’s COVID-19 vaccination programme
It added that protection given by any vaccine is not 100 per cent and "vaccine-breakthrough" infections can occur, with the emergency and spread of new variants due to mutations.
"The detection of asymptomatic to mild infections locally with the Delta variant does not indicate a lack of protection," it said, adding that the findings are in keeping with global evidence that mRNA vaccines have a "high level" of protection against symptomatic and severe COVID-19 disease.
The committee said both mRNA vaccines were approved by multiple international regulatory bodies. Their manufacturers have also "publicly released" detailed study protocols, as well as openly published their findings to be scrutinised by the scientific community after peer review.
Detailed assessments of the vaccines by regulatory bodies such as the US Food and Drug Administration and the UK Medicines & Healthcare Products Regulatory Agency have also been publicly published.
"In sum, the PSAR-authorised mRNA vaccines have reliable scientific evidence that they are safe and effective, and the (committee) continues to strongly recommend that medically eligible persons should be vaccinated with them," it said.
READ: Pfizer-BioNTech, Moderna and Sinovac: A look at three key COVID-19 vaccines
SINOVAC PROTECTION AGAINST NEW VARIANTS STILL UNKNOWN
The committee said the Sinovac COVID-19 vaccine has yet to meet the requirements for a PSAR authorisation by the HSA. Additional safety and quality data required to meet the standards of the evaluation are still pending.
The Sinovac vaccine, which is developed by China's Sinovac Biotech, has shown variable protection across multiple studies carried out internationally, said the committee.
The most complete analysis of the vaccine showed an efficacy of 51 per cent on per-protocol analysis. However, its protection against newer variants such as the Delta variant and under real-world conditions remain unknown, the committee added.
READ: Sinovac COVID-19 vaccine to be allowed in Singapore under special access route after WHO approval
The Sinovac COVID-19 vaccine was approved for emergency use by the World Health Organization last Tuesday. The WHO recommended that it be used for people aged 18 years and older.
As such, the Sinovac vaccine is currently not an option for children and adolescents globally nor in Singapore under the special access route.
"It is critical that medical professionals do not spread unsubstantiated and unscientific information.
"The public has a right to expect medical professionals to give advice based on fact and not on unproven assertions," said the committee.
It also reminded members of the public to rely on reputable sources of scientific and medical information, as well as verify opinions shared by others against such sources.
'No evidence' inactivated virus vaccines more efficacious against COVID-19 variants than mRNA ones: Singapore expert committee
COVID-19 vaccination at Tanjong Pagar, Singapore (4)
A person receiving a dose of the COVID-19 vaccine at Tanjong Pagar Community Centre on Jan 27, 2021. (Photo: Jeremy Long)
Bookmark
SINGAPORE: There is "no evidence" that inactivated virus vaccines demonstrate higher efficacy against COVID-19 variants than messenger RNA (mRNA)-based vaccines, said the government-appointed expert committee on COVID-19 vaccination in Singapore.
"We have noted social media messages asserting that the mRNA COVID-19 vaccines are ineffective against (variants of concern) and that inactivated virus COVID-19 vaccines would provide superior protection," said the committee in a media release on Monday (Jun 7).
The two vaccines currently used in Singapore's national vaccination programme - Pfizer-BioNTech and Moderna - are based on mRNA technology. Both were recommended by the expert committee and authorised by the Health Science Authority (HSA) under the Pandemic Special Access Route (PSAR).
The committee said its assessment, based on a continual review of data and evidence, remains that the authorised mRNA vaccines are "safe and highly effective" and continue to show protection against the virus variants.
"The inactivated virus COVID-19 vaccines have variable protection and there is currently no evidence to suggest that inactivated virus vaccines demonstrate higher vaccine efficacy against (variants of concerns) than the mRNA vaccines," said the committee.
The Sinovac vaccine, of which Singapore has 200,000 doses, uses inactivated viruses.
Monday's clarification by the expert committee follows a similar statement less than three weeks ago, in response to an open letter by 12 doctors that had called for children to be given COVID-19 vaccines other than mRNA ones over fears of "unknown and unstudied" long-term side effects.
Eleven of the 12 doctors later retracted their statements.
AUTHORISED VACCINES "SAFE AND EFFECTIVE" AGAINST COVID-19
Both the Pfizer-BioNTech and Moderna vaccines have shown to be highly efficacious, especially in protecting against severe COVID-19 disease and hospitalisation, said the committee.
It said the high efficacy of the vaccines was demonstrated in Phase 3 clinical trials, and further supported with data from real-world rollouts of the vaccines, including in the United States, the UK and Israel.
This also includes protection against key COVID-19 variants such as the Alpha and Beta variants, which were the predominant strains circulating in these countries. The Alpha variant, B117, was first detected in the UK while the Beta variant, B1351, was first detected in South Africa.
Infographic Greek names for COVID-19 variants B1617
The committee added that despite concerns over the Delta variant, which was first detected in India, data showed that the mRNA vaccines continue to be effective.
It cited a study in the UK, which showed that two doses of the Pfizer-BioNTech COVID-19 vaccine gave about 88 per cent protection against symptomatic COVID-19, even with the Delta variant.
"While further studies are required before a definitive conclusion can be made, the available data globally indicates that substantial protection is preserved," said the committee.
Commentary: Misinformation threatens Singapore’s COVID-19 vaccination programme
It added that protection given by any vaccine is not 100 per cent and "vaccine-breakthrough" infections can occur, with the emergency and spread of new variants due to mutations.
"The detection of asymptomatic to mild infections locally with the Delta variant does not indicate a lack of protection," it said, adding that the findings are in keeping with global evidence that mRNA vaccines have a "high level" of protection against symptomatic and severe COVID-19 disease.
The committee said both mRNA vaccines were approved by multiple international regulatory bodies. Their manufacturers have also "publicly released" detailed study protocols, as well as openly published their findings to be scrutinised by the scientific community after peer review.
Detailed assessments of the vaccines by regulatory bodies such as the US Food and Drug Administration and the UK Medicines & Healthcare Products Regulatory Agency have also been publicly published.
"In sum, the PSAR-authorised mRNA vaccines have reliable scientific evidence that they are safe and effective, and the (committee) continues to strongly recommend that medically eligible persons should be vaccinated with them," it said.
READ: Pfizer-BioNTech, Moderna and Sinovac: A look at three key COVID-19 vaccines
SINOVAC PROTECTION AGAINST NEW VARIANTS STILL UNKNOWN
The committee said the Sinovac COVID-19 vaccine has yet to meet the requirements for a PSAR authorisation by the HSA. Additional safety and quality data required to meet the standards of the evaluation are still pending.
The Sinovac vaccine, which is developed by China's Sinovac Biotech, has shown variable protection across multiple studies carried out internationally, said the committee.
The most complete analysis of the vaccine showed an efficacy of 51 per cent on per-protocol analysis. However, its protection against newer variants such as the Delta variant and under real-world conditions remain unknown, the committee added.
READ: Sinovac COVID-19 vaccine to be allowed in Singapore under special access route after WHO approval
The Sinovac COVID-19 vaccine was approved for emergency use by the World Health Organization last Tuesday. The WHO recommended that it be used for people aged 18 years and older.
As such, the Sinovac vaccine is currently not an option for children and adolescents globally nor in Singapore under the special access route.
"It is critical that medical professionals do not spread unsubstantiated and unscientific information.
"The public has a right to expect medical professionals to give advice based on fact and not on unproven assertions," said the committee.
It also reminded members of the public to rely on reputable sources of scientific and medical information, as well as verify opinions shared by others against such sources.