-
IP addresses are NOT logged in this forum so there's no point asking. Please note that this forum is full of homophobes, racists, lunatics, schizophrenics & absolute nut jobs with a smattering of geniuses, Chinese chauvinists, Moderate Muslims and last but not least a couple of "know-it-alls" constantly sprouting their dubious wisdom. If you believe that content generated by unsavory characters might cause you offense PLEASE LEAVE NOW! Sammyboy Admin and Staff are not responsible for your hurt feelings should you choose to read any of the content here. The OTHER forum is HERE so please stop asking.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
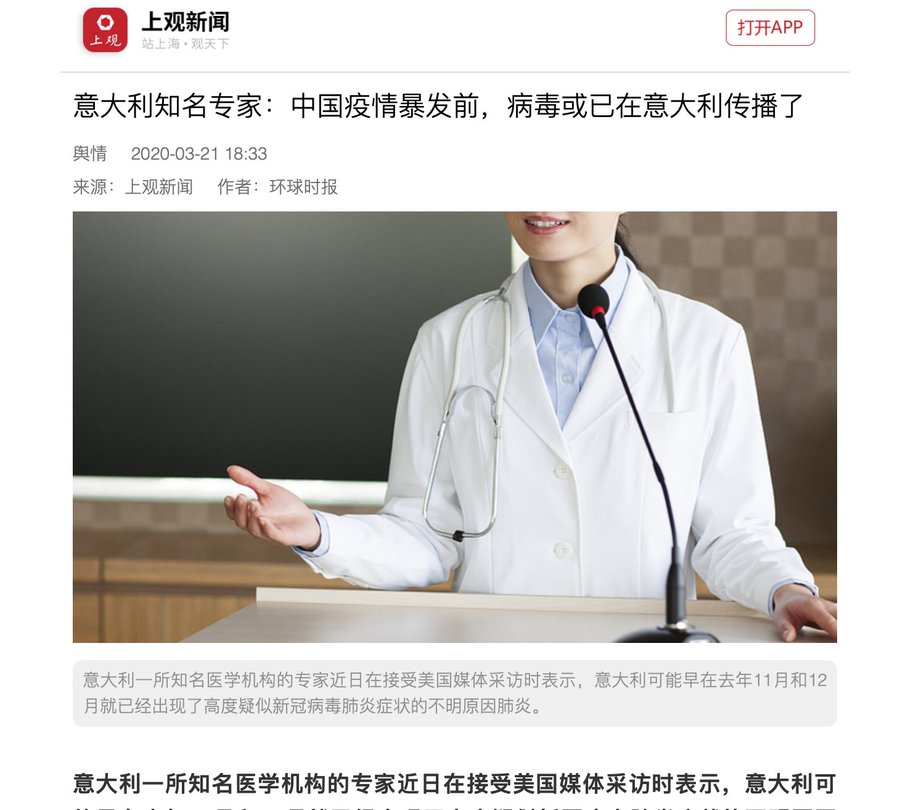
[COVID-19 Virus] The PRC Situation Thread
- Thread starter laksaboy
- Start date
- Joined
- Oct 5, 2018
- Messages
- 17,479
- Points
- 113
Allow yours truly to give his two cents,
so many dead in Europe, probably South America and North America next, do you think these people will just let their people die and not pursue the matter?
Coronavirus is ‘China’s Chernobyl’ And The World Is Paying The Price
https://saraacarter.com/coronavirus-is-chinas-chernobyl-and-the-world-is-paying-the-price
so many dead in Europe, probably South America and North America next, do you think these people will just let their people die and not pursue the matter?
Coronavirus is ‘China’s Chernobyl’ And The World Is Paying The Price
https://saraacarter.com/coronavirus-is-chinas-chernobyl-and-the-world-is-paying-the-price
- Joined
- Sep 17, 2008
- Messages
- 2,954
- Points
- 113
- Joined
- Sep 17, 2008
- Messages
- 2,954
- Points
- 113
- Joined
- Jun 27, 2018
- Messages
- 30,014
- Points
- 113
About time. Ah tiong land getting sued..hope the reparations will be great for the countries that suffer.
- Joined
- Oct 5, 2018
- Messages
- 17,479
- Points
- 113
patient interviews with those exhibiting symptoms such as fever were simplified, and blood tests to detect antibodies produced during infection were discontinued.
https://www.japantimes.co.jp/news/2...rs-manipulated-china-xi-jinping/#.XnqlsogzY2w
https://www.japantimes.co.jp/news/2...rs-manipulated-china-xi-jinping/#.XnqlsogzY2w
- Joined
- Jun 27, 2018
- Messages
- 30,014
- Points
- 113
Ah tiong land civil war? Will Cantoland gain independence?
- Joined
- Jun 27, 2018
- Messages
- 30,014
- Points
- 113
China's coronavirus supplies are being rejected — how do we ensure quality in a pandemic?
 PHOTO Several countries have reported faults with Chinese-made medical equipment imported to fight COVID-19. REUTERS VIA CNSPHOTO
PHOTO Several countries have reported faults with Chinese-made medical equipment imported to fight COVID-19. REUTERS VIA CNSPHOTO
China's had a rough few months.
As the emerging superpower entered 2020, a coronavirus outbreak in the industrial hub of Wuhan swept the nation, then the world, which crippled the global economy in turn.
By March, things began to slowly turn around, as China's drastic lockdown measures appear to have slowed the country's infection rates.
This has placed China in a position to supply sorely needed medical products as coronavirus has shuttered production capacity elsewhere.
Chinese state media Xinhua reported in early March that China was producing 116 million masks daily, about 12 times as many as a month prior, according to official data.
But China's efforts to help haven't gone smoothly, as several countries have reported faults with Chinese-made supplies.
This began with Spain's recall of about 58,000 inaccurate rapid COVID-19 test kits late last week, and Turkey also casting aside a number of sample test kits that were faulty.
This was then followed last Saturday by a Dutch recall of some 600,000 face masks that didn't provide an airtight seal.
So, in a time of supply scarcity during a global pandemic, how do countries ensure the medical supplies they import actually work?
How did faulty equipment get to Europe?
 PHOTO All products that are sold in the EU single market must carry "CE" certification. WIKIMEDIA COMMONS
PHOTO All products that are sold in the EU single market must carry "CE" certification. WIKIMEDIA COMMONS
China's Shenzhen Bioeasy Biotechnology was identified as the manufacturer of Spain's faulty test kits, which local health authorities said had an accuracy rate of about 30 per cent.
Spanish health authorities said they bought Bioeasy's kits as they had European Union standard certification, which is known by its French acronym, CE.
In a statement via WeChat, Bioeasy stated its coronavirus-related products, which include the rapid test kits supplied to Spain, received a CE certificate on March 12.
However, CE certification is mostly self-declared by manufacturers, as it is their responsibility to choose between self-certification or whether to seek an EU-approved independent verifier.
@bidatzi: The external certificate simply certifies that the company has a quality management system. The other one is just BioEasy itself *saying* that their tests comply with applicable standards.
In Australia, Bioeasy's products are not listed on the Register of Therapeutic Goods (ARTG), nor has the company been granted an official vendor licence from China's National Administration of Medical Products, according to a tweet from the Spanish Embassy in China.
In response, Bioeasy said its crown series was not sold in China and was "only used for scientific purposes".
Bioeasy manager Zhu Hai denied the company's test kits had a low accuracy rate in a statement to the South China Morning Post, adding that a "more detailed explanation would be given via official Chinese Government channels".
When asked to comment on the quality concerns on Monday, China's Ministry of Foreign Affairs spokeswoman Hua Chunying said "some masks" ordered by the Dutch "were not suitable for medical staff in intensive care units".
The Chinese Commerce Ministry followed up on Wednesday with a statement compelling all medical product exporters to provide extra documentation showing their products met the standards of its export destination, and were officially registered in China.
Bioeasy initially declined an interview with the ABC, but later shared a test validation report from researchers at Chile's Desarollo University, showing its Fluorescence Antigen rapid test attained 96.1 per cent diagnostic accuracy.
Are there universal standards for PPE or test kits?
 PHOTO This N95 face mask is highly sought after by health systems across the globe. ABC NEWS: PATRICK STONE
PHOTO This N95 face mask is highly sought after by health systems across the globe. ABC NEWS: PATRICK STONE
Technically, there is a global mask standard from the International Standards Organisation (ISO), which outlines the test used to assess a mask's resistance to blood and bodily fluids (including that of small liquid droplets).
However, ISO has stated the test does not "address the performance of the medical face mask's design, construction, interfaces or other factors which may affect the overall protection offered by the medical face mask and its operation".
To determine whether a product is safe to use, health professionals are obligated to look at regulations in their country or at a supranational level for blocs such as the EU.
For example, the Australian Register of Therapeutic Goods has a database of approved medical items for distribution, and that database is updated by the regulatory body Therapeutic Goods Administration (TGA).
On its website, the TGA has specified an exemption for the "importation, manufacture and supply" of COVID-19 test kits to accredited pathology laboratories even if they haven't undergone TGA assessment, and it has also published a list of tests it has approved.
A TGA spokesperson told the ABC via email the body "encourages" all Australians searching for equipment to look at "medical devices included in the ARTG".
Ian Burgess, chief executive of the Medical Technology Association of Australia, told the ABC that Australian medical product manufacturers were "in a position of strength" to respond to COVID-19.
This is to ensure that medical-grade masks such as N95 are available for those who need them the most, such as health workers in the US, which has become the world's latest coronavirus epicentre.
New Yorkers have recently been told to wear face coverings outside of the home, while the White House is considering implementing that rule across the US.
Steve Csiszar, chief executive at Med-Con — Australia's only manufacturer of medical face masks — told the ABC it was critical for people to find masks that meet official standards, otherwise there would be "a false sense of security".
Prior to the pandemic, Med-Con's masks made up 5 per cent of Australia's domestic supply, but the military has since been drafted to ramp up production.
Mr Csiszar said Australia had lost considerable homegrown manufacturing capacity to countries with cheap labour, which has consequences if medical manufacturers cost-cut further.
 PHOTO Manufacturers need to be stringent about non-contamination when putting masks together. REUTERS: UMIT BEKTAS
PHOTO Manufacturers need to be stringent about non-contamination when putting masks together. REUTERS: UMIT BEKTAS
"Quite frankly, I'd be very dubious about any low-cost manufacturer having their products tested to standard," he said.
Additional reporting by Samuel Yang.

China's had a rough few months.
As the emerging superpower entered 2020, a coronavirus outbreak in the industrial hub of Wuhan swept the nation, then the world, which crippled the global economy in turn.
By March, things began to slowly turn around, as China's drastic lockdown measures appear to have slowed the country's infection rates.
This has placed China in a position to supply sorely needed medical products as coronavirus has shuttered production capacity elsewhere.
Chinese state media Xinhua reported in early March that China was producing 116 million masks daily, about 12 times as many as a month prior, according to official data.
But China's efforts to help haven't gone smoothly, as several countries have reported faults with Chinese-made supplies.
This began with Spain's recall of about 58,000 inaccurate rapid COVID-19 test kits late last week, and Turkey also casting aside a number of sample test kits that were faulty.
This was then followed last Saturday by a Dutch recall of some 600,000 face masks that didn't provide an airtight seal.
So, in a time of supply scarcity during a global pandemic, how do countries ensure the medical supplies they import actually work?
How did faulty equipment get to Europe?

China's Shenzhen Bioeasy Biotechnology was identified as the manufacturer of Spain's faulty test kits, which local health authorities said had an accuracy rate of about 30 per cent.
Spanish health authorities said they bought Bioeasy's kits as they had European Union standard certification, which is known by its French acronym, CE.
In a statement via WeChat, Bioeasy stated its coronavirus-related products, which include the rapid test kits supplied to Spain, received a CE certificate on March 12.
However, CE certification is mostly self-declared by manufacturers, as it is their responsibility to choose between self-certification or whether to seek an EU-approved independent verifier.
@bidatzi: The external certificate simply certifies that the company has a quality management system. The other one is just BioEasy itself *saying* that their tests comply with applicable standards.
In Australia, Bioeasy's products are not listed on the Register of Therapeutic Goods (ARTG), nor has the company been granted an official vendor licence from China's National Administration of Medical Products, according to a tweet from the Spanish Embassy in China.
In response, Bioeasy said its crown series was not sold in China and was "only used for scientific purposes".
Bioeasy manager Zhu Hai denied the company's test kits had a low accuracy rate in a statement to the South China Morning Post, adding that a "more detailed explanation would be given via official Chinese Government channels".
When asked to comment on the quality concerns on Monday, China's Ministry of Foreign Affairs spokeswoman Hua Chunying said "some masks" ordered by the Dutch "were not suitable for medical staff in intensive care units".
The Chinese Commerce Ministry followed up on Wednesday with a statement compelling all medical product exporters to provide extra documentation showing their products met the standards of its export destination, and were officially registered in China.
Bioeasy initially declined an interview with the ABC, but later shared a test validation report from researchers at Chile's Desarollo University, showing its Fluorescence Antigen rapid test attained 96.1 per cent diagnostic accuracy.
The ABC was unable to independently verify the data shared."The results show that this antigen test is a very good quality test kit," the company told the ABC in an email.
Are there universal standards for PPE or test kits?

Technically, there is a global mask standard from the International Standards Organisation (ISO), which outlines the test used to assess a mask's resistance to blood and bodily fluids (including that of small liquid droplets).
However, ISO has stated the test does not "address the performance of the medical face mask's design, construction, interfaces or other factors which may affect the overall protection offered by the medical face mask and its operation".
To determine whether a product is safe to use, health professionals are obligated to look at regulations in their country or at a supranational level for blocs such as the EU.
For example, the Australian Register of Therapeutic Goods has a database of approved medical items for distribution, and that database is updated by the regulatory body Therapeutic Goods Administration (TGA).
On its website, the TGA has specified an exemption for the "importation, manufacture and supply" of COVID-19 test kits to accredited pathology laboratories even if they haven't undergone TGA assessment, and it has also published a list of tests it has approved.
A TGA spokesperson told the ABC via email the body "encourages" all Australians searching for equipment to look at "medical devices included in the ARTG".
The World Heath Organisation has also published a list of recommendations for PPE usage amid COVID-19."Manufacturers of medical devices must be able to provide evidence that demonstrates they have used appropriate design, development and production systems to the manufacture of their medical device," the spokesperson said
Ian Burgess, chief executive of the Medical Technology Association of Australia, told the ABC that Australian medical product manufacturers were "in a position of strength" to respond to COVID-19.
Until now, the US Centres for Disease Control and Prevention, like the World Health Organisation, has advised that healthy people do not need to wear a mask."Medical technology companies see the COVID-19 response as a national duty and have been working around the clock together to fight this unprecedented virus," Mr Burgess said.
This is to ensure that medical-grade masks such as N95 are available for those who need them the most, such as health workers in the US, which has become the world's latest coronavirus epicentre.
New Yorkers have recently been told to wear face coverings outside of the home, while the White House is considering implementing that rule across the US.
Steve Csiszar, chief executive at Med-Con — Australia's only manufacturer of medical face masks — told the ABC it was critical for people to find masks that meet official standards, otherwise there would be "a false sense of security".
Prior to the pandemic, Med-Con's masks made up 5 per cent of Australia's domestic supply, but the military has since been drafted to ramp up production.
Mr Csiszar said Australia had lost considerable homegrown manufacturing capacity to countries with cheap labour, which has consequences if medical manufacturers cost-cut further.

"Quite frankly, I'd be very dubious about any low-cost manufacturer having their products tested to standard," he said.
He said that because masks use synthetic materials, masks not to standard also run the risk of introducing other pathogens that may be travelling with the raw materials, or the product."You may as well just have a handkerchief or a piece of toilet paper over your face — it's useless."
The ABC has contacted the researchers cited by Bioeasy for comment."Faulty masks are a two-fold thing: they don't work, but they could also cause more damage by introducing third-party problems that weren't there before."
Additional reporting by Samuel Yang.
- Joined
- Jun 27, 2018
- Messages
- 30,014
- Points
- 113
- Joined
- Oct 19, 2008
- Messages
- 28,387
- Points
- 113
Talking cockWith 17000 new cases, US overtakes China
About time. Ah tiong land getting sued..hope the reparations will be great for the countries that suffer.
Yes.
China is to blame for all the lockdowns and what not.
If they had come clean and reported the 200 million cases they have and that only a few thousand deaths thus showing that the mortality rate is low then we would NOT have had to have lockdowns.
It is all China's fault. They also wayang and do their own lockdown and made the Wuhan virus sound more serious. Scare the world.
You want to blame someone , blame China. Not your local doctor/prostitute lah.
- Joined
- Jun 27, 2018
- Messages
- 30,014
- Points
- 113
Help wanted: Jobless China workers await relief as COVID-19 hits economy
Chinese brokerage Zhongtai Securities last week estimated joblessness at 20.5 percent
Chinese brokerage Zhongtai Securities last week estimated joblessness at 20.5 percent. (Photo: AFP/Hector Retamal)
30 Apr 2020 11:51AM
Bookmark
SHANGHAI: Life is never easy for China's nearly 300 million migrant workers, but with the coronavirus zapping jobs at a historic clip, unemployed factory labourer Wei Guikun is at his wits' end.
Since March, Wei has wandered in search of work from his home in eastern Shandong province - where virus lockdowns stranded him for weeks - to factories in China's southern coastal regions, and now back north to Shanghai.
READ: China's Q1 GDP posts first decline on record as COVID-19 shuts down economy
But the pandemic has paralysed overseas export demand and the 29-year-old has faced only rejection or low-ball pay on his roughly 3,500km odyssey.
Workers wait in line to collect salaries in Shanghai
Workers wait in line to collect salaries in Shanghai. (Photo: AFP/Hector Retamal)
The global pandemic will force millions worldwide to spend Friday's International Workers Day in unemployment and uncertainty.
But perhaps nowhere is the pressure felt as keenly as in the world's second-largest economy, where the Communist Party has long staked its legitimacy on delivering jobs and prosperity in return for public acquiescence to its political monopoly.
The pandemic has paralysed overseas export demand and hit China's factories hard
The pandemic has paralysed overseas export demand and hit China's factories hard. (Photo: AFP/Hector Retamal)
"My fellow workers will definitely be angry (if their livelihoods suffer)," Wei said, pulling a wheeled suitcase down a seemingly endless avenue in a sprawling Shanghai factory district after another rejection.
"I've met fellow workers from Shandong, Henan, and Heilongjiang. They're all like that."
The situation raises the spectre of millions of angry citizens potentially protesting over their misfortune.
At this point, no one expects mass worker unrest in tightly controlled China, but joblessness is soaring after the economy shrank for the first time in decades in the first three months of the year.
GRAVE CONCERN
UBS Securities said this week that perhaps 80 million jobs have been lost in services, industry and construction and more than 10 million other jobs could evaporate in export sectors as orders cease and businesses avoid large worker gatherings because of lingering virus fears.
READ: Commentary: When China sneezes, the world economy catches a COVID-19 cold
"China's labour market pressure may be the most challenging since the late 1990s and early 2000s," UBS said.
Chinese brokerage Zhongtai Securities last week estimated joblessness at 20.5 per cent, or around 70 million workers - far exceeding that of the 2008-09 global financial crisis and three times official estimates.
Zhao Chang sits in an employment agency in Shanghai waiting for a lift to his new factory job
Zhao Chang sits in an employment agency in Shanghai waiting for a lift to his new factory job. (Photo: AFP/Hector Retamal)
But in an indication of the sensitivity of jobs data in China, Zhongtai later retracted the report, apparently under official pressure.
Even if still employed, an estimated 250 million workers will lose 10-50 per cent of earnings this year, the Economist Intelligence Unit said.
But despite an array of stimulus measures such as business-tax breaks and liquidity injections for markets, UBS said help for idled workers has been "very limited even though the labour market hit has been substantial".
Geoffrey Crothall of China Labor Bulletin (CLB), which tracks Chinese labour strikes and workplace abuses, said "clearly there is grave concern in the central government".
"However, so far the relief measures have done very little to actually benefit workers," he added.
CLB already has tracked scores of small-scale work-related protests in the country since the virus erupted.
"There are going to be more and more (Chinese) workers not getting paid, and I think you will see a commensurate rise in protests," Crothall said.
"Much depends on whether people are willing to gather in large groups again."
DEBT WORRIES
Various reasons for the inaction have been put suggested, including fear of over-stimulating the economy and creating a debt hangover like that built up in 2008-09, which China is still struggling to reduce.
The virus also postponed the annual national parliament session, where major policies are enacted.
The Communist Party has long staked its legitimacy on delivering jobs and prosperity in return for
The Communist Party has long staked its legitimacy on delivering jobs and prosperity in return for public acquiescence to its political monopoly. (Photo: AFP/Hector Retamal)
Originally scheduled for early March, it will now begin on May 22, state media reported on Wednesday.
The central government last week indicated its concern about workers, urging greater help for rural poor and migrants, including extending unemployment benefits to them for the first time.
But Crothall said such benefits are so low and difficult to obtain that they are "really not going to help".
But other measures are on the table.
Apparently fearing a backlash, the central government has ordered cities to make it easier for migrants to obtain residency where they work, opening access to local social services like schooling and medical care.
Until then, life is on hold for Wei, who had hoped to earn enough to marry soon but returned this week to Shandong, still jobless.
"If I still can't find a job or salaries are low, it will definitely affect my ability to marry and I'll be worried."
Source: AFP/ad
Chinese brokerage Zhongtai Securities last week estimated joblessness at 20.5 percent
Chinese brokerage Zhongtai Securities last week estimated joblessness at 20.5 percent. (Photo: AFP/Hector Retamal)
30 Apr 2020 11:51AM
Bookmark
SHANGHAI: Life is never easy for China's nearly 300 million migrant workers, but with the coronavirus zapping jobs at a historic clip, unemployed factory labourer Wei Guikun is at his wits' end.
Since March, Wei has wandered in search of work from his home in eastern Shandong province - where virus lockdowns stranded him for weeks - to factories in China's southern coastal regions, and now back north to Shanghai.
READ: China's Q1 GDP posts first decline on record as COVID-19 shuts down economy
But the pandemic has paralysed overseas export demand and the 29-year-old has faced only rejection or low-ball pay on his roughly 3,500km odyssey.
Workers wait in line to collect salaries in Shanghai
Workers wait in line to collect salaries in Shanghai. (Photo: AFP/Hector Retamal)
The global pandemic will force millions worldwide to spend Friday's International Workers Day in unemployment and uncertainty.
But perhaps nowhere is the pressure felt as keenly as in the world's second-largest economy, where the Communist Party has long staked its legitimacy on delivering jobs and prosperity in return for public acquiescence to its political monopoly.
The pandemic has paralysed overseas export demand and hit China's factories hard
The pandemic has paralysed overseas export demand and hit China's factories hard. (Photo: AFP/Hector Retamal)
"My fellow workers will definitely be angry (if their livelihoods suffer)," Wei said, pulling a wheeled suitcase down a seemingly endless avenue in a sprawling Shanghai factory district after another rejection.
"I've met fellow workers from Shandong, Henan, and Heilongjiang. They're all like that."
The situation raises the spectre of millions of angry citizens potentially protesting over their misfortune.
At this point, no one expects mass worker unrest in tightly controlled China, but joblessness is soaring after the economy shrank for the first time in decades in the first three months of the year.
GRAVE CONCERN
UBS Securities said this week that perhaps 80 million jobs have been lost in services, industry and construction and more than 10 million other jobs could evaporate in export sectors as orders cease and businesses avoid large worker gatherings because of lingering virus fears.
READ: Commentary: When China sneezes, the world economy catches a COVID-19 cold
"China's labour market pressure may be the most challenging since the late 1990s and early 2000s," UBS said.
Chinese brokerage Zhongtai Securities last week estimated joblessness at 20.5 per cent, or around 70 million workers - far exceeding that of the 2008-09 global financial crisis and three times official estimates.
Zhao Chang sits in an employment agency in Shanghai waiting for a lift to his new factory job
Zhao Chang sits in an employment agency in Shanghai waiting for a lift to his new factory job. (Photo: AFP/Hector Retamal)
But in an indication of the sensitivity of jobs data in China, Zhongtai later retracted the report, apparently under official pressure.
Even if still employed, an estimated 250 million workers will lose 10-50 per cent of earnings this year, the Economist Intelligence Unit said.
But despite an array of stimulus measures such as business-tax breaks and liquidity injections for markets, UBS said help for idled workers has been "very limited even though the labour market hit has been substantial".
Geoffrey Crothall of China Labor Bulletin (CLB), which tracks Chinese labour strikes and workplace abuses, said "clearly there is grave concern in the central government".
"However, so far the relief measures have done very little to actually benefit workers," he added.
CLB already has tracked scores of small-scale work-related protests in the country since the virus erupted.
"There are going to be more and more (Chinese) workers not getting paid, and I think you will see a commensurate rise in protests," Crothall said.
"Much depends on whether people are willing to gather in large groups again."
DEBT WORRIES
Various reasons for the inaction have been put suggested, including fear of over-stimulating the economy and creating a debt hangover like that built up in 2008-09, which China is still struggling to reduce.
The virus also postponed the annual national parliament session, where major policies are enacted.
The Communist Party has long staked its legitimacy on delivering jobs and prosperity in return for
The Communist Party has long staked its legitimacy on delivering jobs and prosperity in return for public acquiescence to its political monopoly. (Photo: AFP/Hector Retamal)
Originally scheduled for early March, it will now begin on May 22, state media reported on Wednesday.
The central government last week indicated its concern about workers, urging greater help for rural poor and migrants, including extending unemployment benefits to them for the first time.
But Crothall said such benefits are so low and difficult to obtain that they are "really not going to help".
But other measures are on the table.
Apparently fearing a backlash, the central government has ordered cities to make it easier for migrants to obtain residency where they work, opening access to local social services like schooling and medical care.
Until then, life is on hold for Wei, who had hoped to earn enough to marry soon but returned this week to Shandong, still jobless.
"If I still can't find a job or salaries are low, it will definitely affect my ability to marry and I'll be worried."
Source: AFP/ad
Similar threads
- Replies
- 28
- Views
- 1K
- Replies
- 0
- Views
- 224
- Replies
- 2
- Views
- 231
- Replies
- 0
- Views
- 243