- Joined
- Aug 29, 2008
- Messages
- 27,211
- Points
- 113
Risk of Severe Coronavirus Linked to Neanderthal Genes From 60,000 Years Ago
TESSA KOUMOUNDOUROS
1 OCTOBER 2020
Long ago, in the south of Europe, modern humans and Neanderthals had at least one encounter that resulted in children. While dalliances between our two species are now well documented, no one could possibly have foreseen how grimly they would impact our world 60,000 years later.
A resulting stretch of Neanderthal DNA spread far through our populations as it was passed down through the generations, even while the Neanderthals themselves became extinct. Around 50 percent of the people in South Asia and 16 percent of people in Europe now carry this length of DNA, which scientists have now linked to the most severe form of COVID-19.
According to the new research, those who have this genetic inheritance are three times more likely to require mechanical ventilation once they contract the virus, explains evolutionary anthropologist Hugo Zeberg from the Max Planck Institute for Evolutionary Anthropology in Germany.
Scientists have been scrambling to understand what makes some people more vulnerable to SARS-COV-2 than others. The disease has now taken over a million human lives.
While pre-existing underlying conditions and contributing social inequalities explain a large part of our vulnerability, there still stubbornly remains a significant portion of people who are young and healthy yet inexplicably end up with severe respiratory problems, whereas their equally healthy peers only experience the mildest symptoms.
Zeberg and geneticist Svante Pääbo from the Okinawa Institute of Science and Technology in Japan analysed genetic data from 3,199 hospitalised COVID-19 patients and saw certain gene variants on chromosome 3 are found together in the population more often than if they were random mutations.
Such a long length of DNA, spanning six genes and adding up to 49.4 thousand bases being passed down together, suggests this variation was introduced into the human genome together, meaning it was inherited.
Previous research linked this gene region to patients that had a severe reaction to SARS-CoV-2, requiring hospitalisation.
So Zeberg and Pääbo investigated our extinct human relatives to see where this length of genes came from. They found none of these specific gene variants in the Denisovan genome, and a few of them were found in two Neanderthals from Siberia. But a Neanderthal from Croatia shared the most similarities.
These results are "compatible with this Neanderthal being closer to the majority of the Neanderthals who contributed DNA to present-day people," the researchers wrote.
Zeberg and Pääbo calculated that it was very unlikely this combination of genes came from a shared ancestor of both humans and Neanderthals, meaning they were introduced when our two species interbred.
We don't yet know why this snippet of chromosome 3 increases the risk of severe illness.
"This is something that we and others are now investigating as quickly as possible," explained Pääbo.
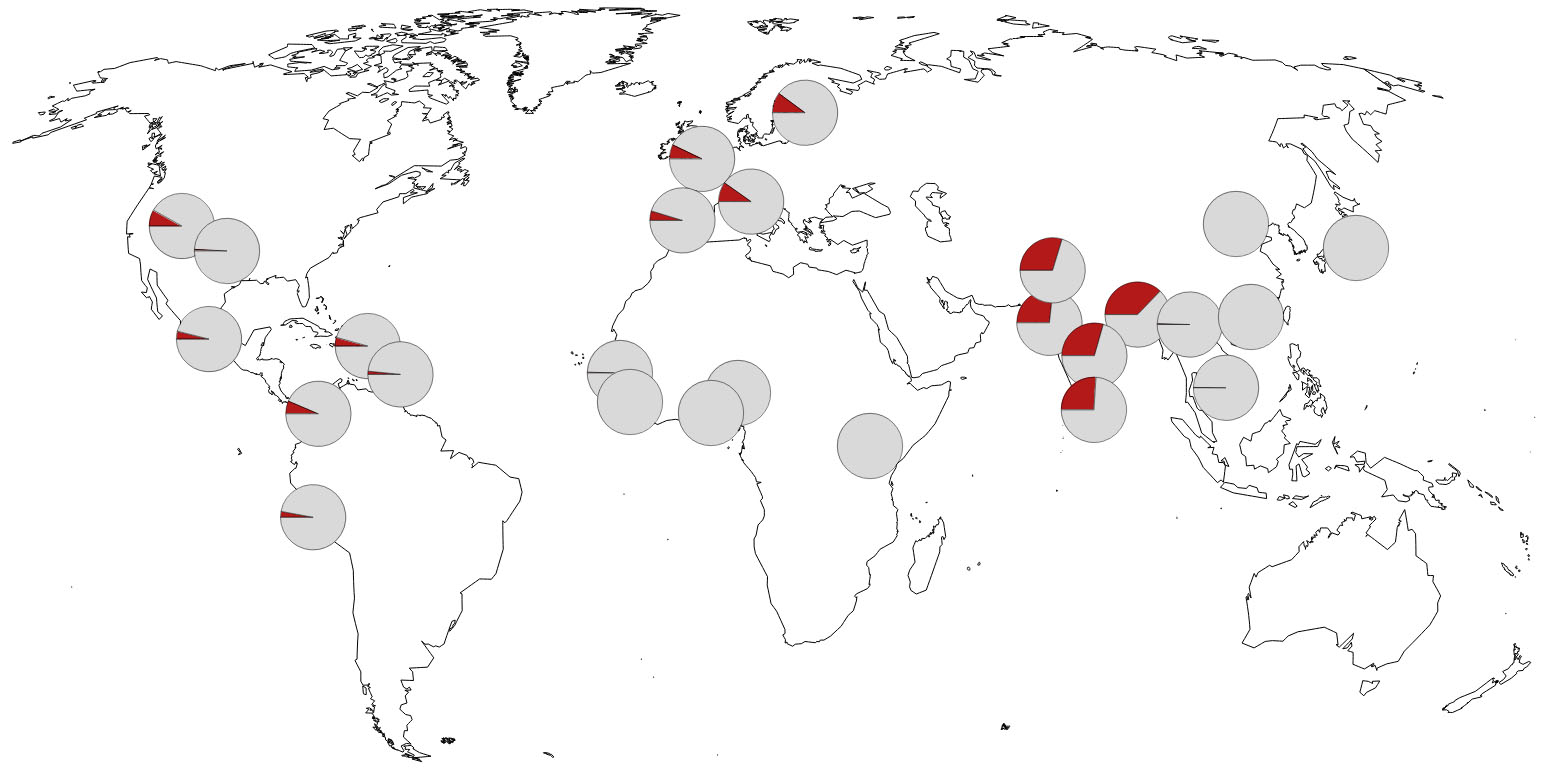 Distribution and prevalence (pie charts) of the Neanderthal genetic variants. (Zeberg et al., Nature, 2020)
Distribution and prevalence (pie charts) of the Neanderthal genetic variants. (Zeberg et al., Nature, 2020)
The team suspect that in the past these genes may have proved an advantage for some people - perhaps against another pathogen. A previous study hinted Neanderthal DNA may have provided protection against ancient viruses.
This may explain why this now unfortunate variant of chromosome 3 is prevalent in some populations, like in Bangladesh, where 63 percent of people have it, but it's almost absent in others, such as Africa.
This distribution could explain why people of Bangladeshi descent in the UK are twice as likely to die from COVID-19 compared to the rest of the population.
"It is striking that the genetic heritage from Neanderthals has such tragic consequences during the current pandemic," said Pääbo.
Last week, another team identified a potential immune mechanism that may also contribute to severe coronavirus cases.
However these genetic pieces of the coronavirus puzzle end up fitting together, it is important to remember that environmental factors also play a large role in whether we even contract the disease in the first place - and that's something we have control over today.
This research was published in Nature.
TESSA KOUMOUNDOUROS
1 OCTOBER 2020
Long ago, in the south of Europe, modern humans and Neanderthals had at least one encounter that resulted in children. While dalliances between our two species are now well documented, no one could possibly have foreseen how grimly they would impact our world 60,000 years later.
A resulting stretch of Neanderthal DNA spread far through our populations as it was passed down through the generations, even while the Neanderthals themselves became extinct. Around 50 percent of the people in South Asia and 16 percent of people in Europe now carry this length of DNA, which scientists have now linked to the most severe form of COVID-19.
According to the new research, those who have this genetic inheritance are three times more likely to require mechanical ventilation once they contract the virus, explains evolutionary anthropologist Hugo Zeberg from the Max Planck Institute for Evolutionary Anthropology in Germany.
Scientists have been scrambling to understand what makes some people more vulnerable to SARS-COV-2 than others. The disease has now taken over a million human lives.
While pre-existing underlying conditions and contributing social inequalities explain a large part of our vulnerability, there still stubbornly remains a significant portion of people who are young and healthy yet inexplicably end up with severe respiratory problems, whereas their equally healthy peers only experience the mildest symptoms.
Zeberg and geneticist Svante Pääbo from the Okinawa Institute of Science and Technology in Japan analysed genetic data from 3,199 hospitalised COVID-19 patients and saw certain gene variants on chromosome 3 are found together in the population more often than if they were random mutations.
Such a long length of DNA, spanning six genes and adding up to 49.4 thousand bases being passed down together, suggests this variation was introduced into the human genome together, meaning it was inherited.
Previous research linked this gene region to patients that had a severe reaction to SARS-CoV-2, requiring hospitalisation.
So Zeberg and Pääbo investigated our extinct human relatives to see where this length of genes came from. They found none of these specific gene variants in the Denisovan genome, and a few of them were found in two Neanderthals from Siberia. But a Neanderthal from Croatia shared the most similarities.
These results are "compatible with this Neanderthal being closer to the majority of the Neanderthals who contributed DNA to present-day people," the researchers wrote.
Zeberg and Pääbo calculated that it was very unlikely this combination of genes came from a shared ancestor of both humans and Neanderthals, meaning they were introduced when our two species interbred.
We don't yet know why this snippet of chromosome 3 increases the risk of severe illness.
"This is something that we and others are now investigating as quickly as possible," explained Pääbo.

The team suspect that in the past these genes may have proved an advantage for some people - perhaps against another pathogen. A previous study hinted Neanderthal DNA may have provided protection against ancient viruses.
This may explain why this now unfortunate variant of chromosome 3 is prevalent in some populations, like in Bangladesh, where 63 percent of people have it, but it's almost absent in others, such as Africa.
This distribution could explain why people of Bangladeshi descent in the UK are twice as likely to die from COVID-19 compared to the rest of the population.
"It is striking that the genetic heritage from Neanderthals has such tragic consequences during the current pandemic," said Pääbo.
Last week, another team identified a potential immune mechanism that may also contribute to severe coronavirus cases.
However these genetic pieces of the coronavirus puzzle end up fitting together, it is important to remember that environmental factors also play a large role in whether we even contract the disease in the first place - and that's something we have control over today.
This research was published in Nature.

